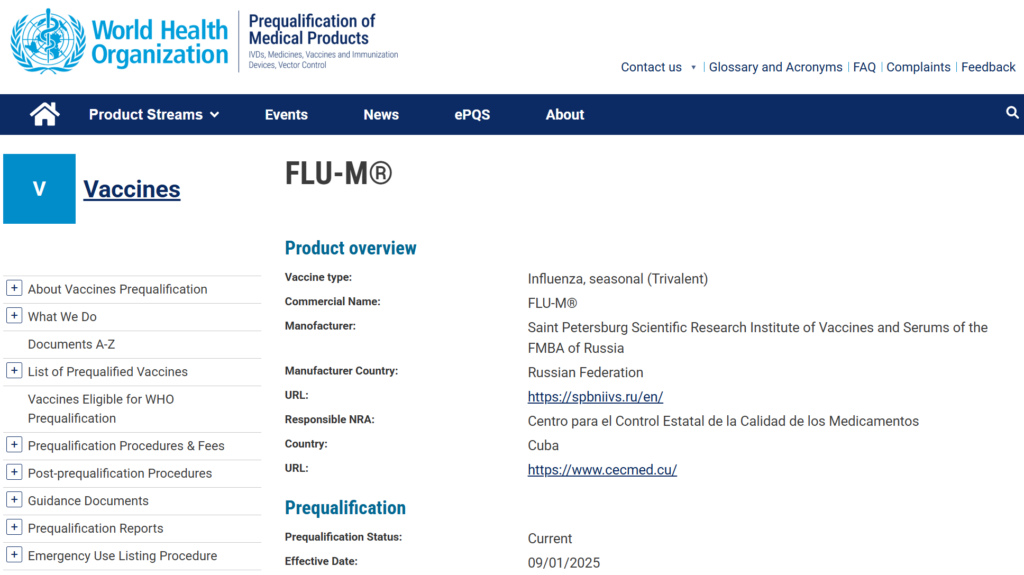
We are thrilled to share an incredible achievement from two of our esteemed members, SPbSRIVS (Saint Petersburg Institute of Vaccines and Serums FMBA of Russia) and Instituto Latinoamericano de Biotecnología MECHNIKOV (Nicaragua). Their work has resulted in the successful World Health Organization prequalification of the FLU-M influenza vaccine, marking a monumental milestone in the fight against influenza as it is now one of only two WHO-prequalified influenza vaccines in the Latin America and Caribbean region!
FLU-M is now the first Russian influenza vaccine to receive WHO prequalification and is officially recommended for purchase by UN agencies such as the Pan American Health Organization Revolving Fund and UNICEF.
FLU-M, a trivalent inactivated split vaccine, is designed to prevent influenza without the use of adjuvants. The rigorous WHO prequalification process reaffirmed its high quality, safety, and efficacy, meeting the most stringent international standards. This thorough assessment resulted in FLU-M joining the ranks of other globally recognized influenza vaccines, including Vaxigrip (Sanofi), GC Flu (GC Biopharma (GC녹십자)) and Serinflu (Abbott Biologicals).
At DCVMN, we are proud to celebrate this remarkable achievement and applaud SPbSRIVS and Instituto MECHNIKOV, S.A. for their dedication to advancing public health!
For more details on the WHO prequalification and vaccine product: https://extranet.who.int/prequal/vaccines/p/flu-mr
Image from https://extranet.who.int/prequal/vaccines/p/flu-mr