Technology Transfer Training UAT
Technology Transfer
Training
Recognizing the critical role of technology transfers, we have developed the DCVMN Technology Transfer Training Programme. This comprehensive initiative facilitates knowledge exchange and skill development among vaccine manufacturers, focusing on the intricacies of vaccine production and the tech-transfer process. The program features a hybrid training journey, combining online theoretical learning with in-person practical sessions, and culminating in hands-on practice in partner laboratories.

Why Technology
Transfer Training?
Understanding the pivotal role that technology transfers play in enhancing the capabilities of vaccine manufacturers in developing countries, DCVMN has collaborated with leading expert laboratories to create the comprehensive Technology Transfer Training Programme. This programme is designed to provide in-depth education and hands-on experience in vaccine production. It facilitates the exchange of knowledge and best practices among participants, focusing particularly on the intricacies of the technology transfer process and the essential requirements for successful implementation. Through this initiative, we aim to build a robust network of skilled professionals who are well-equipped to return to their companies and share this knowledge to advance their vaccine manufacturing.

This training programme is unique in its international scope, welcoming participants from multiple countries. It provides essential professional exposure and expert guidance on technical mastery, which are crucial for process optimization, development, and the transfer of vaccine knowledge.
With each Training programme, we continually refine and enhance our approach based on active participation and valuable feedback from participants. This iterative process ensures the course structure remains dynamic and effective. Currently, the training is divided into two distinct phases:

Online Phase
Participants are introduced to a 5-week blended course which helps set the foundation for this intensive training and allows participants to interact with their trainers as well as fellow participants. This phase prepares the batch to maximise their learning in the next phase of this training program.
Onsite Phase
This phase involves hands-on training. Participants gain immersive experience with cutting-edge technologies and have the opportunity to consult with subject-matter experts, providing a comprehensive understanding of practical applications. This, in combination with the online phase, prepares our participants to contribute substantially and meaningfully upon their return to their home organisations.
You focus on learning we will take care of the rest
The training fee, flights and accommodation of the attendees are fully covered by DCVMN. For each edition of the training programme, select employees from vaccine manufacturers in developing countries are chosen to participate. The selection process begins with shortlisting companies based on specific criteria, which may vary slightly with each edition. Given the exclusivity of this training opportunity, DCVMN directly contacts manufacturers, except in cases of special partnerships with other institutions, to nominate their employees. Each company is invited to nominate up to three candidates, ideally including one representative from production, quality control (QC), and/or quality assurance (QA), based on the selection criteria given.

To assess the participants’ learning and the programme’s effectiveness, a 3-step evaluation is conducted for each training edition. On the first day, a pre-training test evaluates the participants’ initial understanding. This is followed by a performance evaluation at the completion of the training programme. Finally, a day-90 follow-up assesses job performance outcomes to analyze long-term benefits and the implementation of the training.
Mastering technology transfers is crucial for bridging the time and expense gap in vaccine manufacturing. By building local expertise and capacity, the programme fosters a sustainable supply of vaccines, ultimately contributing to global health security and resilience. The long-term benefits of this training are profound, as they enable countries to become self-reliant in vaccine production, respond more effectively to public health needs, and enhance their ability to combat infectious diseases.

Technology Transfer Training Editions

1st Tech-Transfer Training
June, 2022
The first DCVMN Technology Transfer Training took place in June 2022, in collaboration with Hilleman Laboratories in Singapore. Fifteen participants from seven DCVMN companies from Bangladesh, India, Taipei, and Vietnam were selected to attend this edition.

2nd Tech-Transfer Training
February to March, 2023
The second DCVMN Technology Transfer Training took place from February to March 2023, in collaboration with Hilleman Laboratories in Singapore. Fifteen participants from nine DCVMN companies from Argentina, Brazil, China, India, Senegal, Thailand, and Vietnam were selected to attend this edition.

3rd Tech-Transfer Training
October to November, 2023
The third DCVMN Technology Transfer Training occurred from October to November 2023, in collaboration with Africa CDC. This cohort exclusively featured bright minds from Algeria, Egypt, Senegal, and South Africa. The in-person training, held in Singapore, provided hands-on experience in partnership with Hilleman Laboratories. This edition marked the introduction of a hybrid training format, where a 5-week online training preceded the hands-on component. This recent revamp of the programme received overwhelmingly positive feedback from participants, as it allowed them more time to absorb the course material and refine their skills effectively.

4th Tech-Transfer Training
June to July, 2024
The fourth DCVMN Technology Transfer Training took place from June to July 2024, in collaboration with Hilleman Laboratories in Singapore. This edition maintained the successful hybrid training format introduced in the third edition. Fifteen participants from twelve DCVMN member companies from Bangladesh, China, India, Indonesia, Republic of Korea, Thailand, and Vietnam were selected to attend this edition.
Training Outcome
The primary achievement of each DCVMN technology transfer training is the amplification of knowledge once participants return to their respective companies. The pre-training and post-training evaluation surveys reveal that approximately 70-80% of participants experienced a significant increase in their general knowledge of technology transfer and their understanding of regulatory authority requirements. Following the training, 90% of participants expressed confidence in participating in a tech-transfer project and indicated that they would recommend the training to their colleagues. The high level of improvement on the subject suggests that these areas were previously underemphasized in participants’ knowledge bases, making the training particularly valuable. The willingness of participants to recommend the programme further validates its relevance and quality. Overall, these results highlight the programme’s success in equipping professionals with the necessary skills and knowledge to contribute effectively to tech-transfer projects.

Based on the 90-day post-training evaluation, we observed a significant increase in knowledge dissemination, with a more than fourfold amplification through participant sharing sessions. Upon returning, participants conducted knowledge-sharing sessions, with 42% of these sessions attended by 4-6 people, 33% by 1-3 people, and 17% by more than 7 people.
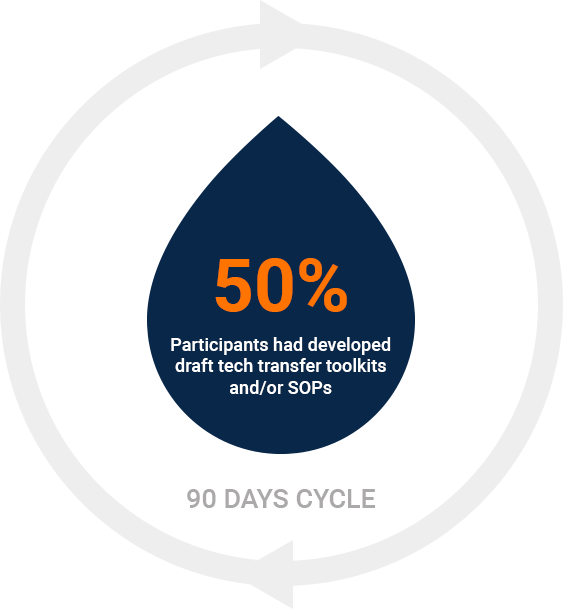
Post 90 days from the training, 50% of the participants had developed draft tech transfer toolkits and/or standard operating procedures (SOPs) for their organizations. Additionally, 29% undertook a tech transfer project, and 21% made improvements to existing tech transfer SOPs, manuals, or toolkits within their companies.These outcomes demonstrate the training’s substantial impact on enhancing organizational capabilities. The creation of toolkits and SOPs indicates a solidification of the learned principles, while the initiation of tech transfer projects and improvements to existing processes reflect the practical application of the training. This ripple effect of knowledge transfer highlights the programme’s effectiveness in not only educating individual participants but also in fostering a culture of continuous improvement and capacity building within their organizations.
These outcomes underscore the programme’s ability to bridge knowledge gaps and equip participants with practical skills. The pre- and post-training evaluations not only demonstrate the immediate impact of the training but also suggest long-term retention and application of the material. The high rate of positive feedback from the day-90 evaluation indicates that participants were able to effectively translate their learning into their professional roles. This level of success not only validates the training methods used but also supports the ongoing development and refinement of the programme to ensure it meets the evolving needs of vaccine manufacturers in developing countries.
Testimonies
I have gained deep insights of what an effective tech-transfer entails through the HL-DCVMN Tech-Transfer Training Programme. I found the hands-on training, lab based equipment demonstration, interactive group discussion sessions, and problem based case study useful.
from India
The programme was incredibly enlightening and provided me with a wealth of knowledge and practical skills in technology transfer, intellectual property, and scientific innovations, that I can immediately apply to my work.
from Brazil
It was a great learning experience and applicable knowledge that I can implement in our system for improvement of technology transfer management. The training course was designed very well and beneficial. It was a good platform to interact with the expert partners, mentors and other participant DCVMN members. Thank you to HL-DCVMN for this opportunity.
from Thailand
Knowledge around tech-transfer within my country South Africa and other LMICs in general, is extremely lacking. I think during COVID-19, we saw the different disparities that existed within the country, and how so many vital technologies were not available for the region, not necessarily only in Africa, but in LMICs in general. As the spotlight is now on the region to try and become self-sustaining in terms of technology and vaccine access and healthcare access in general, I am extremely grateful for the opportunity to learn from the expertise that exists within Singapore and Hilleman Laboratories, and I think that this training will significantly contribute to me being able to play a vital role in ensuring that access and ensuring that the people of my region and my country are able to have access to these lifesaving technologies.
from South africa
The programme was incredibly enlightening and provided me with a wealth of knowledge and practical skills in technology transfer, intellectual property, and scientific innovations, that I can immediately apply to my work.
from Brazil
It was a great learning experience and applicable knowledge that I can implement in our system for improvement of technology transfer management. The training course was designed very well and beneficial. It was a good platform to interact with the expert trainers, mentors and other participant DCVMN members. Thank you to HL-DCVMN for this opportunity.
from Thailand
The comprehensive overview of the entire process from upstream to downstream through to filling will definitely enable my company to speed up our technology transfer plans and launch products more quickly.
from China
My biggest takeaway from the Hilleman-DCVMN Technology Transfer Training Program was being able to fully understand the entire technology transfer flow and all the requirements associated with the process. I’m very grateful to have participated in the course and for the care and attention that DCVMN and Hilleman Laboratories gave us during these two weeks.
from Brazil
DCVMN gave me a great opportunity to attend this training and to dive deeply into the subject of tech-transfer. This training gave me good knowledge on technology transfer and how to plan it systematically. After this training, I will take this knowledge back to my country and adjust what we already have in technology transfer agreements and to have a new way to start new technology plans and ensure successful tech-transfer. My commitment after this training will be how to implement what I learned here to my country and company and to get the best benefit from any technology transfer.
from Egypt
Our Sponsors











