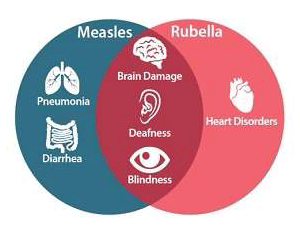
Geneva, 29 September 2019 – A new measles-rubella (MR) vaccine has been prequalified by the World Health Organization (WHO) Biological E (BioE), a leading vaccine manufacturer in India, thanks to a partnership with PATH. Like measles, rubella is known for the long-term health consequences it can wreak on children less than five years old. Given the high risk of death or debilitating complications malnourished children face, the WHO recommends tackling these illnesses together with the MR vaccine. Unfortunately, adequate supply is a challenge.
Securing robust and affordable MR vaccine supply is critical: few diseases are more contagious than measles, and few public health interventions have had such a dramatic and rapid impact as the introduction of the measles vaccine. WHO prequalification, or certification, allows a vaccine to be procured through common subsidized funding mechanisms like Gavi, the Vaccine Alliance, and/or UNICEF. It means a vaccine has achieved WHO’s standards for manufacturing, safety, and efficacy, and it is the result of a vaccine’s development journey that is several years in the making.
PATH partnered with BioE beginning at the research and development phase, providing technical assistance and expertise to improve BioE’s manufacturing process and increase its production capacity. Eventually, we provided input into the design of a new facility dedicated solely to MR vaccine production to ensure a robust supply and supported BioE through the process of WHO prequalification. PATH’s Center for Vaccine Innovation and Access and BioE are guided by principles of accessibility and affordability for the communities who most need vaccines.
More information at: https://path.org/articles/new-measles-rubella-vaccine-those-who-need-it-most/









